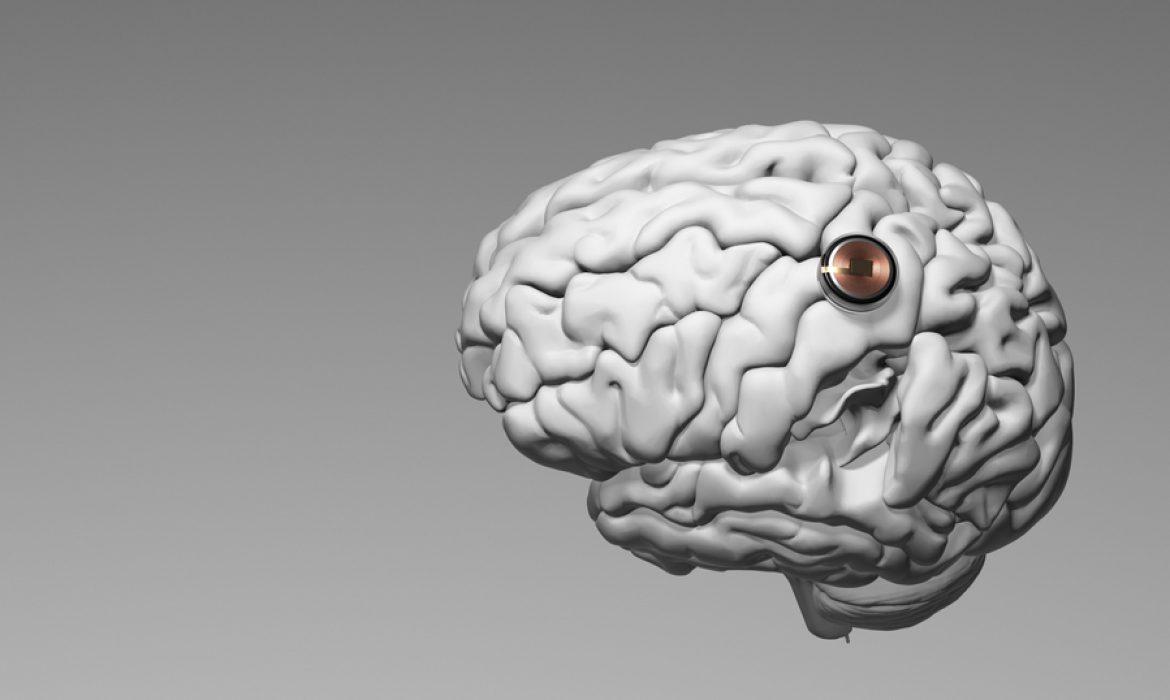
In a monumental stride toward merging technology with the human brain, Elon Musk’s Neuralink has successfully implanted a brain chip into a human for the first time. The procedure, conducted on Sunday, marks a significant moment in the startup’s journey to bring life-changing technology out of the lab and into real-world applications.
Elon Musk Announces Milestone on Social Media Platform X
Elon Musk, billionaire founder of Neuralink, took to his social media platform X on Monday to announce the groundbreaking achievement. The first patient who received the brain chip implant is reportedly showing signs of improvement. Musk emphasized the promising results, stating, “Initial results show promising detection of neuronal splices.” Neuronal spiking, the activity of neurons using electrical and chemical signals, plays a crucial role in transmitting information throughout the brain and body.
Neuralink’s Vision for Human Trials and Beyond
Neuralink received approval from the U.S. Food and Drug Administration (FDA) last year to conduct the first human trial of its brain chip implant. The startup aims to address conditions like paralysis and neurological disorders. The procedure involves a robot surgically placing a brain-computer interface (BCI) implant in the brain region controlling the intention to move. The initial objective is to empower individuals to control a computer cursor or keyboard using their thoughts alone.
Telepathy: Neuralink’s Pioneering Product
Elon Musk unveiled that Neuralink’s inaugural product resulting from this groundbreaking work will be named “Telepathy.” He envisions this technology as a means to enable communication for individuals who have lost the use of their limbs. Musk’s vision extends to a scenario where individuals like Stephen Hawking could communicate faster than traditional methods, such as typing or speaking.
Challenges and Scrutiny Surrounding Neuralink
Despite the milestone, Neuralink has faced scrutiny and challenges. Earlier this month, the company faced fines for violating U.S. Department of Transportation regulations regarding hazardous materials transportation. The company’s valuation, once at $5 billion in June, experienced fluctuations following concerns raised by lawmakers about the safety of Neuralink’s technology.
Neuralink’s journey faced criticism when a monkey died during a 2022 experiment, leading to allegations of rushed market entry, negligent animal deaths, and a federal investigation. The startup received FDA approval for human clinical trials last May, recruiting patients with quadriplegia caused by spinal cord injuries or ALS.
Regulatory Hurdles and a Visionary Future
While Neuralink’s success in implanting a brain chip is significant, regulatory approval is necessary before the technology reaches the broader market. The FDA recognizes the rapidly evolving nature of brain-computer interface devices, highlighting the need for careful evaluation.
Other companies, such as Synchron, are also working on similar neural interface technologies. Neuralink’s achievements have garnered attention, yet the practical applications and general availability of such neural interfaces remain a prospect for the future. The idea of interfaces between the brain and the nervous system holds great potential for aiding individuals with neurological disorders, although widespread adoption may still be years away, given the experimental stage of these technologies.